I Introduction
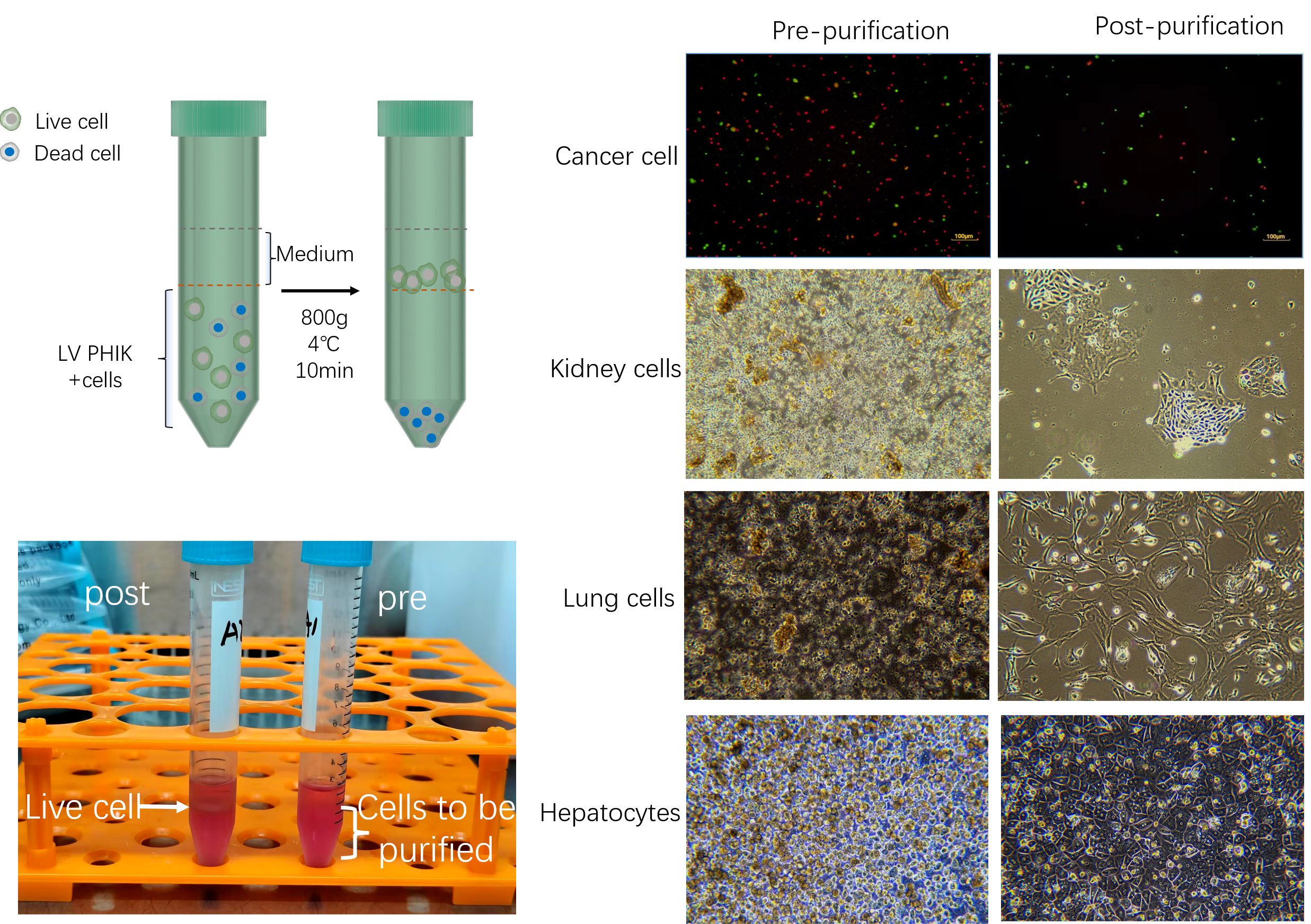
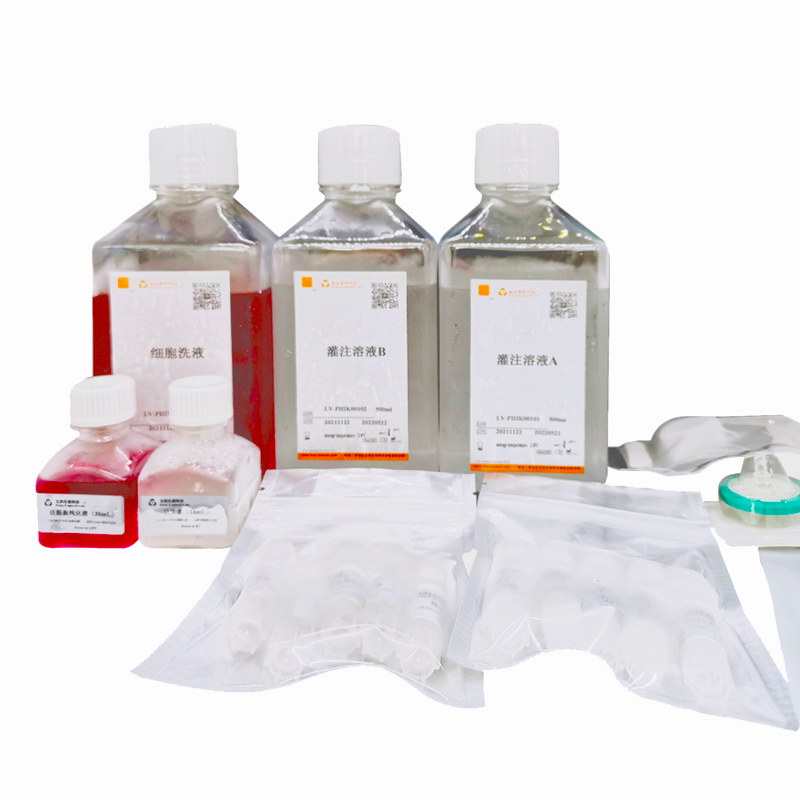
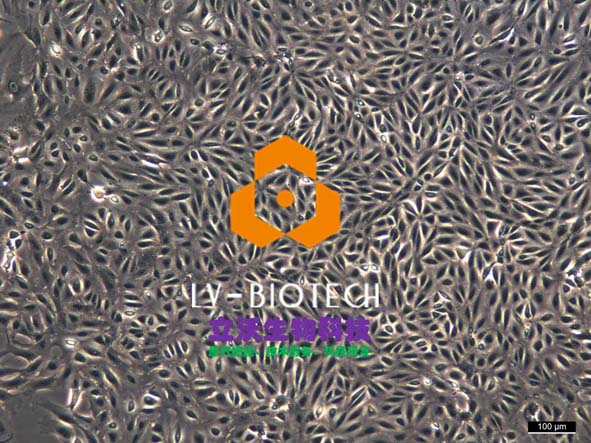
Viable cell purification medium (LV-PHIK00104) can efficiently remove impurities such as dead cells and cell debris, and improve the efficiency of cell recovery through new centrifugal media with excellent biocompatibility and the exclusion of live cells to media. At the same time, this product has the advantages of maintaining normal osmolality of cells, repairing some damaged cells and providing nutrient requirements for cells. After testing, this product can be used in the process of live cell isolation and purification of a variety of primary cells of various species (human, monkey, pig, rabbit, rat, mouse, etc.), including liver cells, vascular endothelial cells, islet beta cells, etc.
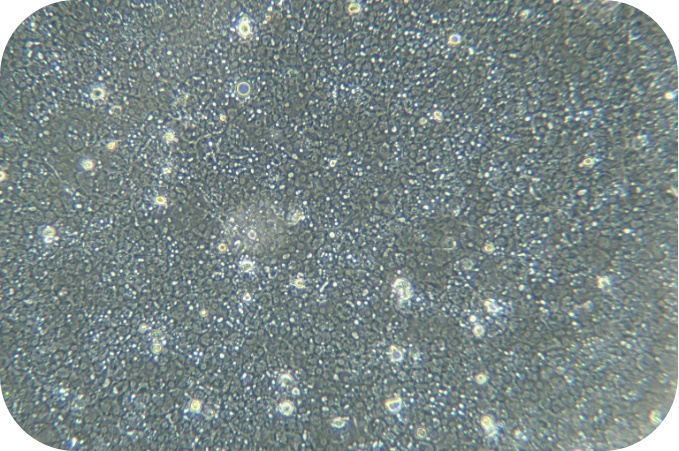
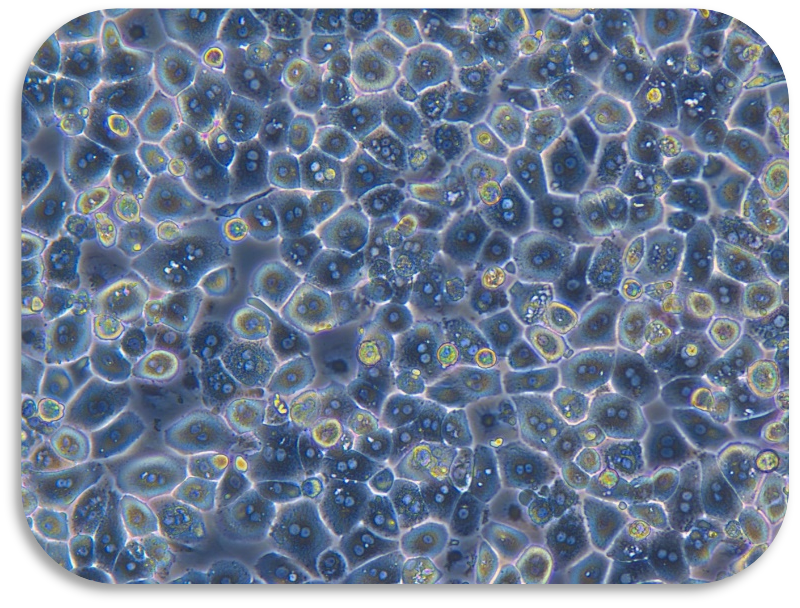
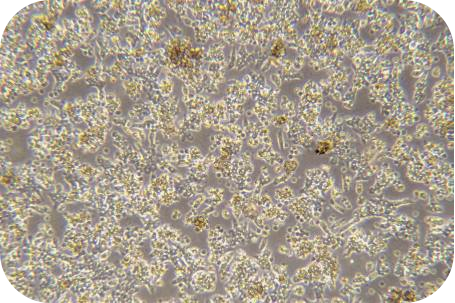
II Cell isolation (hepatocytes as an example)
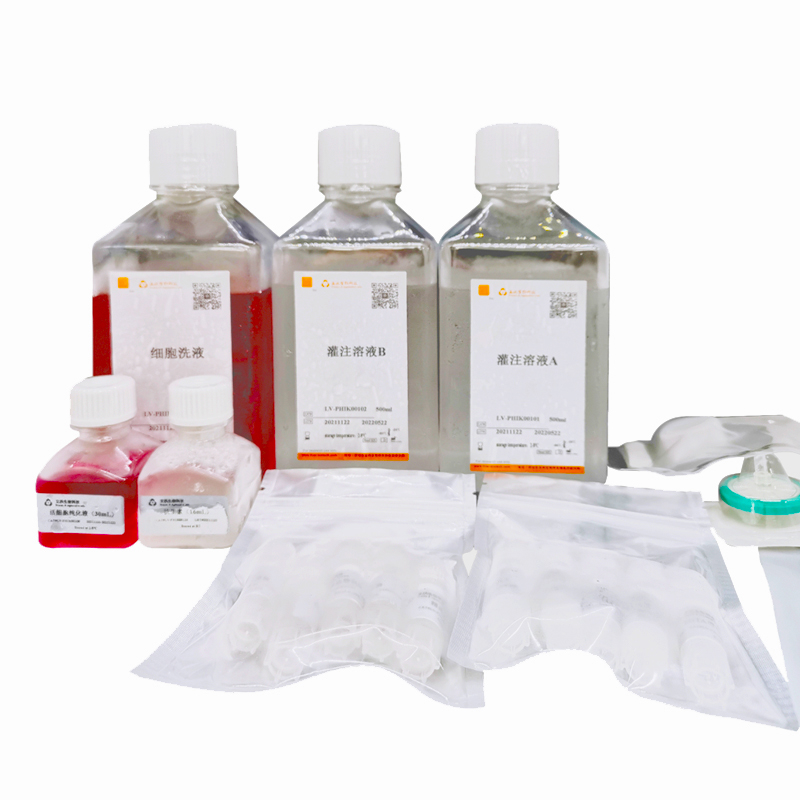
1. Perfuse the liver with two-step collagenase. The specific steps should be based on the conditions of laboratory. Usually, perfusion buffer I (containing EGTA) should be perfused for 10min to 30min, and perfusion buffer II (containing collagenase) preheated at 37 °C should be perfused for 15min to 30min.
2. Stop digestion and disperse cells. Terminate digestion with serum-containing medium. Then pass through a 70 μm cell sieve to obtain a single cell suspension.
3. Wash with washing buffer. Centrifuge of 50× g at 4 °C for 5 min. After that, go supernatant and centrifuge 2 times.
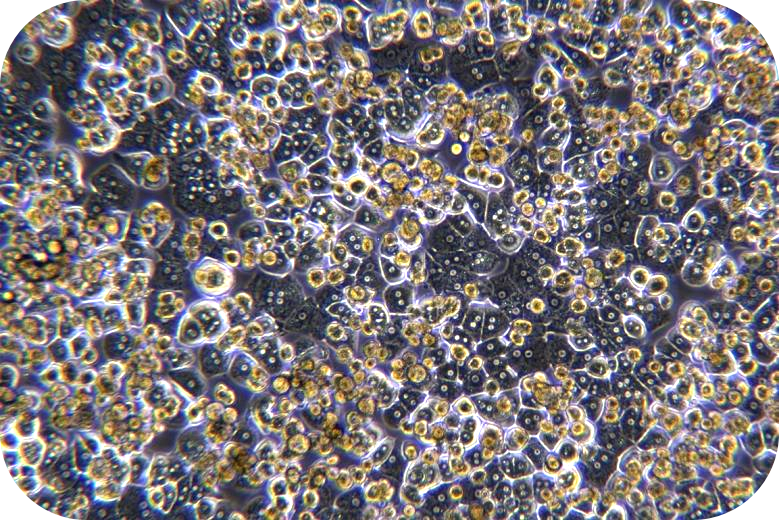
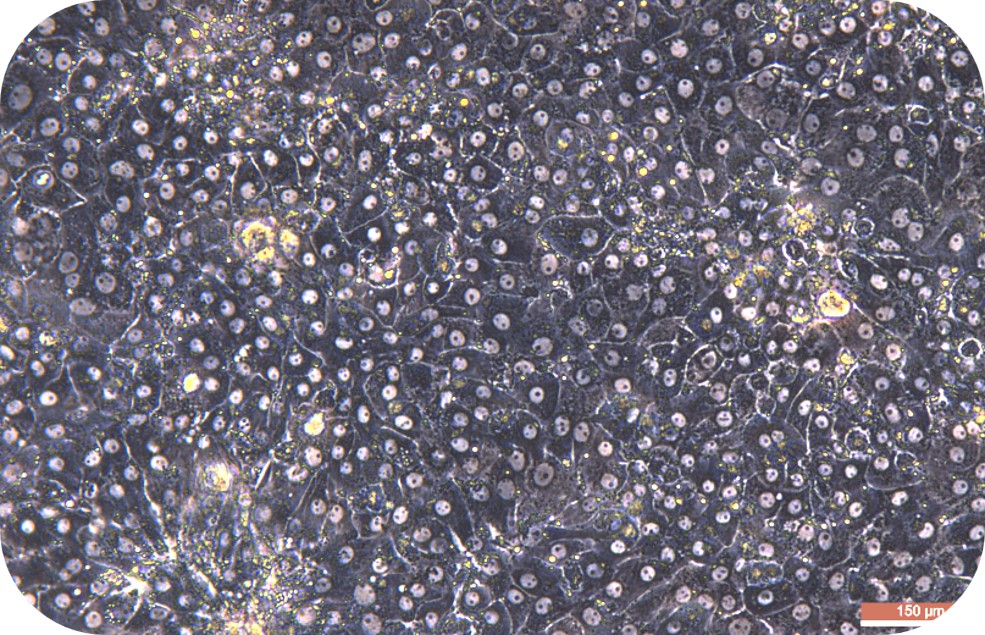
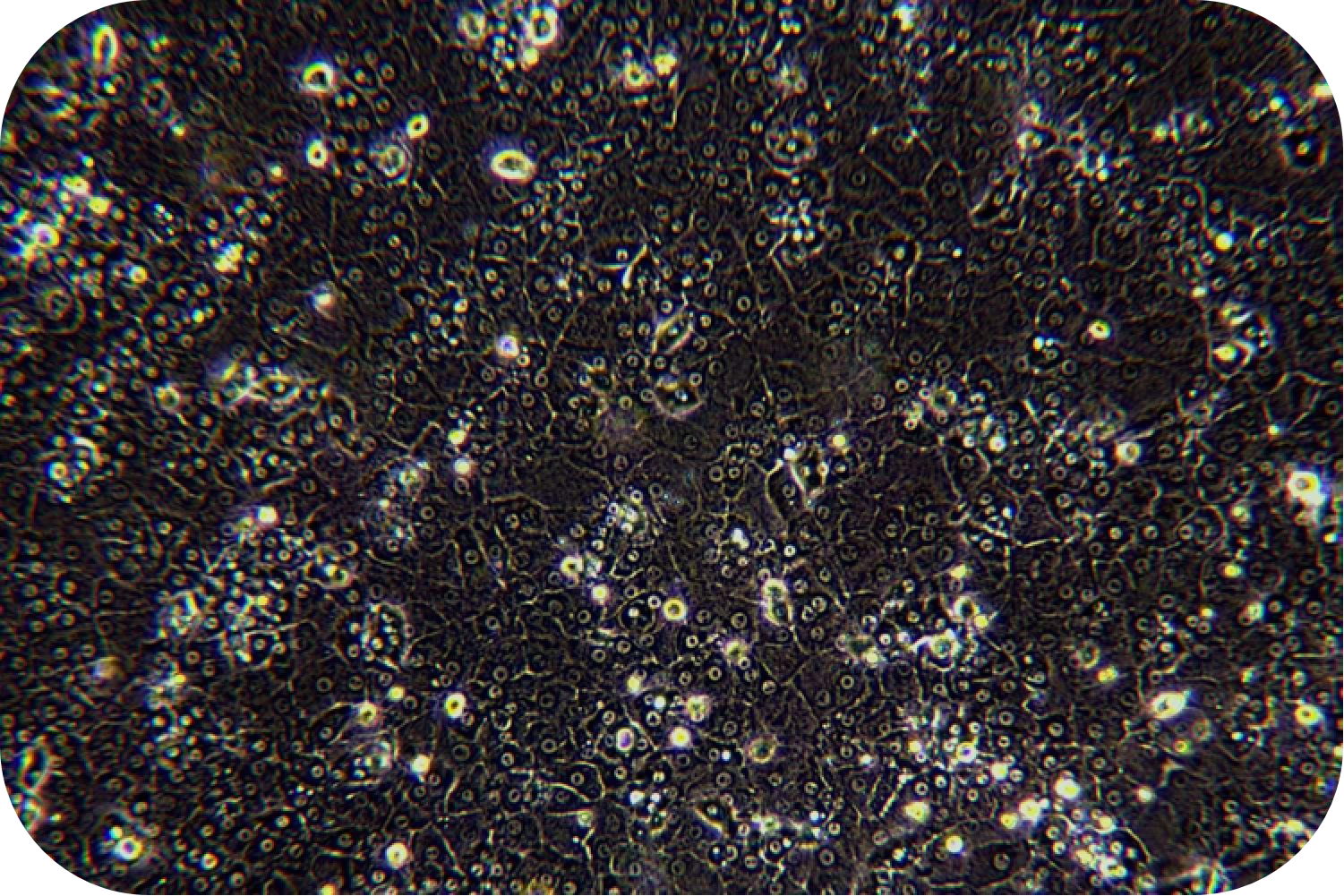
4. Viability assay: resuspend cells, take a small amount of cell suspension, and count with trypan blue exclusion. If cell viability is less than 70%, or does not meet expectations, step of living cell purification can be performed.
5. Cell suspension centrifuged to supernatant. Then centrifuged of 50× g at 4 °C for 5 min. For subsequent purification, 15 mL centrifuge tubes can be used for the total number of cells below 1*108, and 50 m L tubes can be used for the total number of cells above 1*108.
III Steps of Purification
1. Add the purification medium to a centrifuge tube containing the pelleted cells. Resuspend gently the cells with a wide-mouth pipette tip. The recommended amounts of purified medium are shown in the table below:
Total Number | Volume of Purified Medium/mL |
1*106~1*107 | 2~3 |
1*107~1*108 | 3~10 |
1*108~3*108 | 10~15 |
>3*108 | Calculation of the actual total cell volume/108*15mL |
2. Carefully add 2-5 mL of hepatogenic plating medium to the centrifuge tube along the wall (other conventional media are also available). Do not disturb the lower layers. Significant layering can be visible after addition.
3. Centrifuge at 4 °C of 800 × g for 20 min (ascending to 9, downgrading to 1).
4. Take the cell layer between the purified medium and the plating medium and transfer it to a new centrifuge tube. Then, add twice the volume of plating medium (other conventional medium is also available). Centrifuge of 50× g at 4 °C for 5 min once.
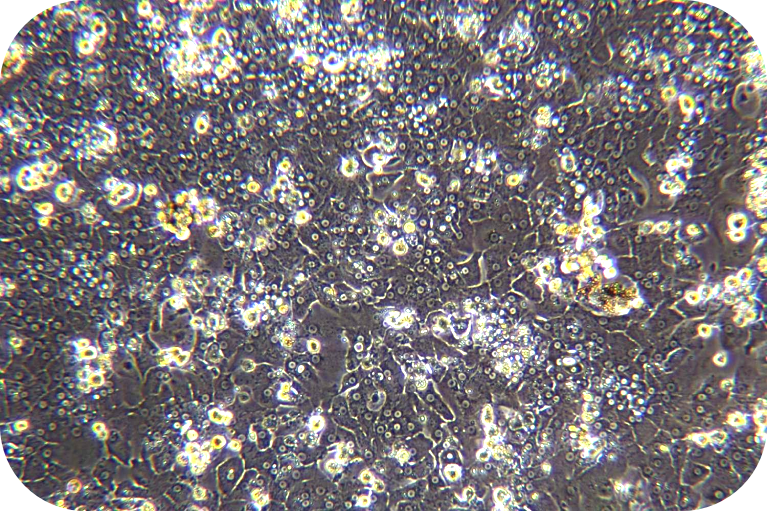
5. Go to supernatant and add the plating medium to resuspend. Take a small amount of cell suspension and count with trypan blue exclusion.
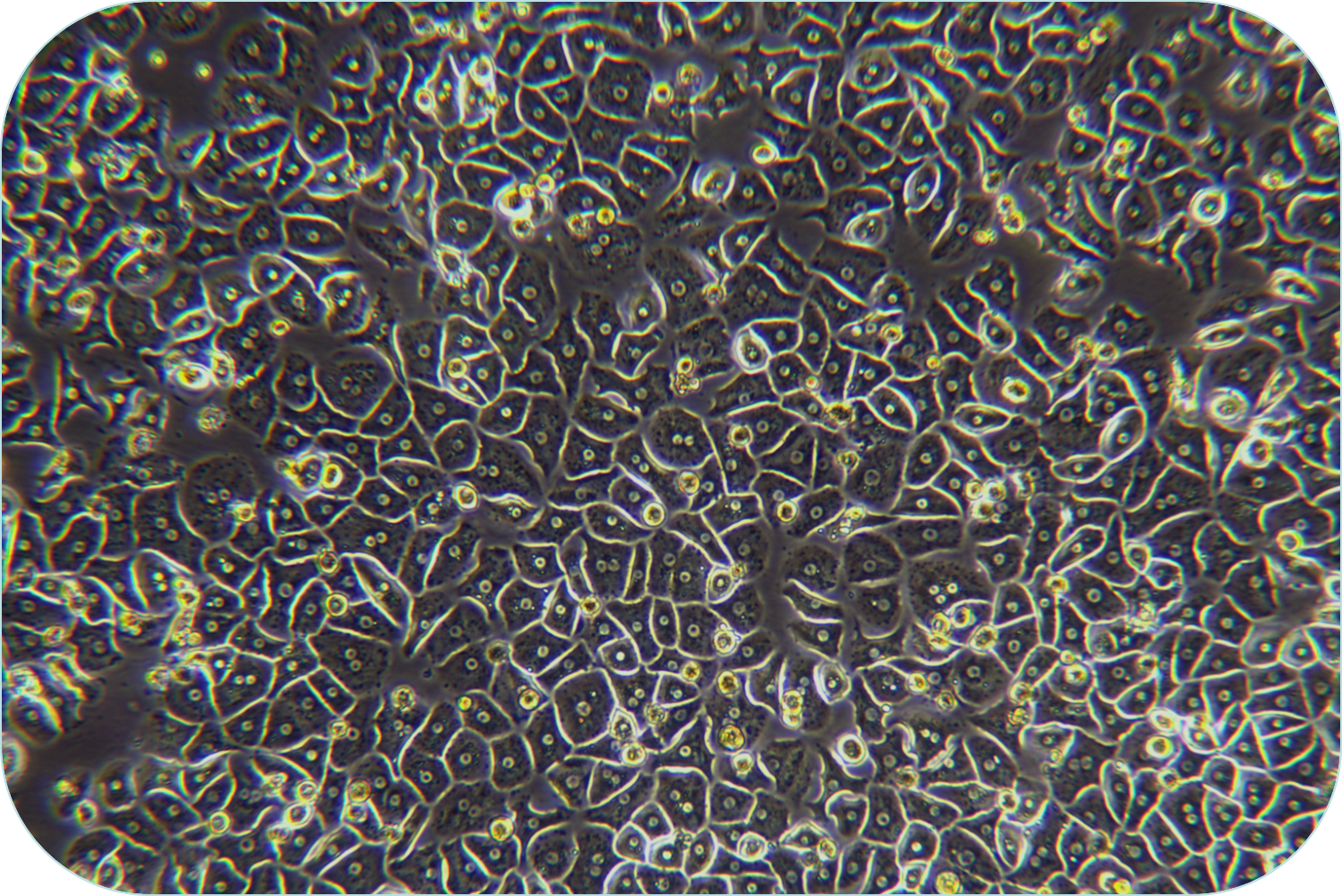
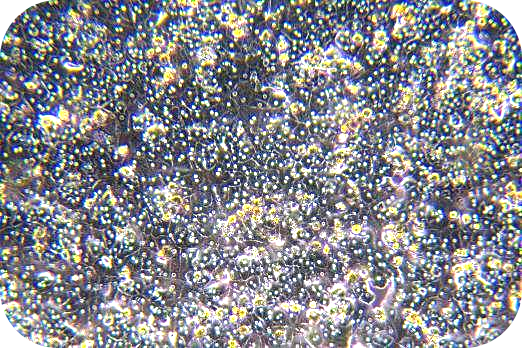
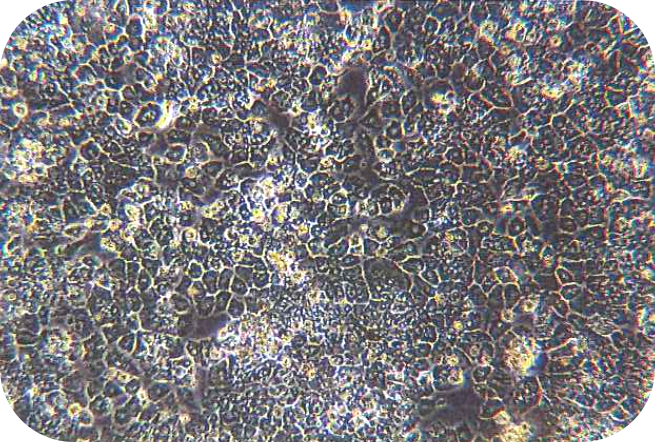
6. Seed liver cells into collagen-coated plates. Seed hepatocytes into collagen-coated plates. The next steps follow the usual operation.
IV Special Instructions
Wide-mouth pipette tip: The 1 mL of tip should be cut off and sterilized to reduce the shearing force of cells.