Drug Toxicology Evaluation Technical Services
I. Introduction to Drug Toxicity
Drug toxicity refers to harmful effects on the body caused by drug exposure at doses exceeding therapeutic levels or during prolonged use, potentially leading to organ dysfunction or tissue damage. Toxicology evaluation is a critical step in drug development, aiming to assess potential risks and ensure drug safety and efficacy. Toxicity may involve multiple organ systems (e.g., liver, kidneys, nervous system), necessitating comprehensive toxicological studies to characterize safety profiles.
II. Detection Models
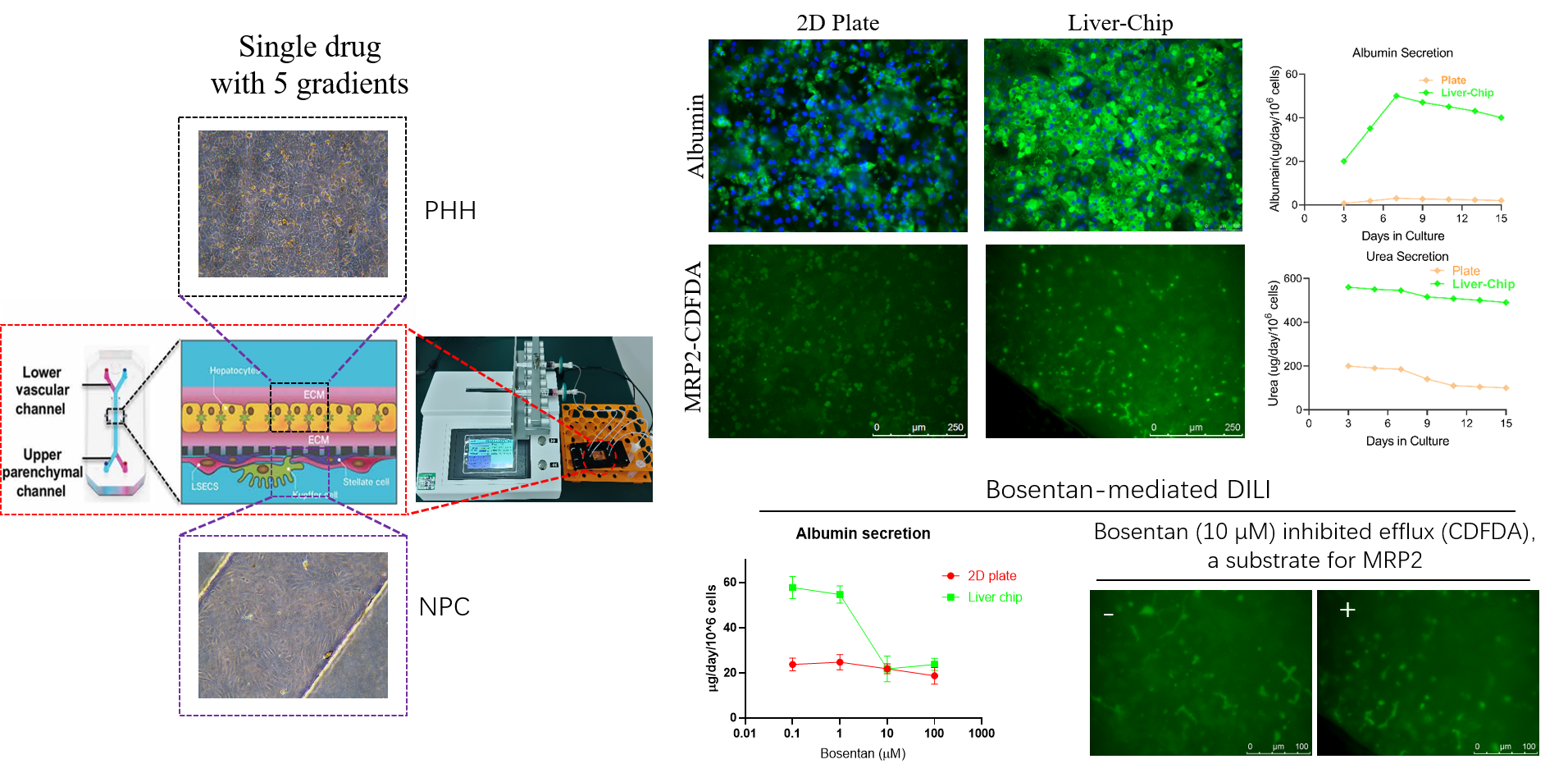
1. Adherent Primary Hepatocytes
Species: Human, monkey, dog, rat, mouse.
Applications:

2. 3D Liver Spheroids (Mini-Liver)

3. Organ-on-a-Chip
III. Service Credentials, Facilities, and Team

Expert Team:
Ph.D. in Virology, Wuhan Institute of Virology, Chinese Academy of Sciences.
14+ years of expertise in liver research.
Principal investigator for 7 national/provincial grants (NSFC, Guangdong DST, Shenzhen STIC).
10+ first/corresponding-author SCI publications.
AIMBE Fellow, National Young Thousand Talents Program awardees, and Shenzhen High-Level Talent Awardees.
Dr. Ming Zhou:
Ph.D. in Virology, Wuhan Institute of Virology, Chinese Academy of Sciences.
14+ years of expertise in liver research.
Principal investigator for 7 national/provincial grants (NSFC, Guangdong DST, Shenzhen STIC).
10+ first/corresponding-author SCI publications.
IV. Case Studies
Case 1: A Dongguan Biopharmaceutical Company
Case 2: A Guangxi Research Institution









