HBV Infection-Related Technical Services
I. Model Overview
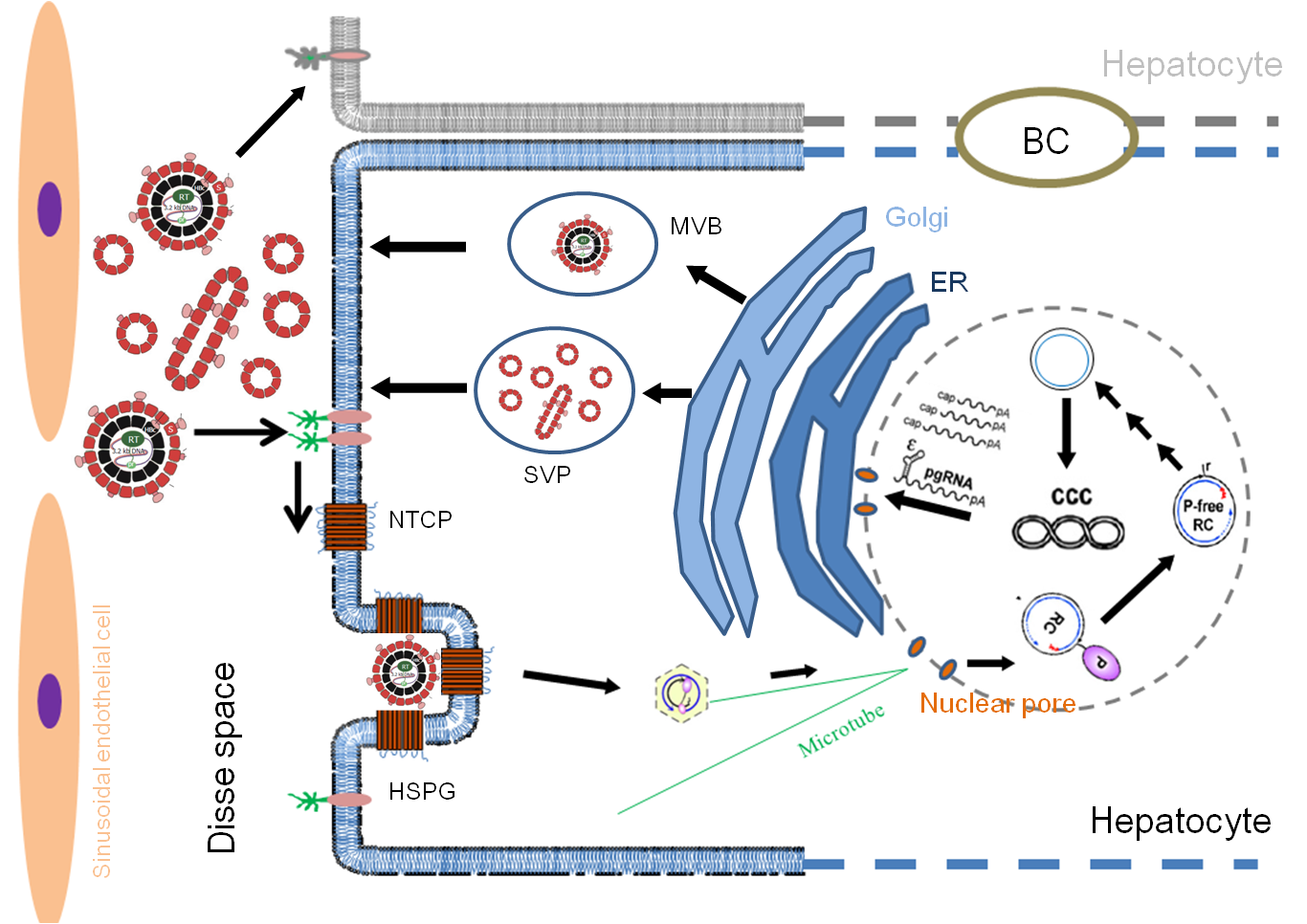
The life cycle of the Hepatitis B Virus (HBV) is a highly coordinated molecular biological process, involving the following key stages and molecular events:
Viral Entry
Formation of Covalently Closed Circular DNA (cccDNA)
Viral Transcription
Nucleocapsid Assembly and Reverse Transcription
Virion Maturation and Secretion
Secondary Infection and Transmission
The complete life cycle of HBV is illustrated below:
HBV Infection Models: Types and Their Advantages/Limitations
1. Primary Hepatocyte Models (Gold Standard)
Human Primary Hepatocytes (PHH) and Tupaia Hepatocytes:
Crab-Eating Monkey Primary Hepatocytes + hNTCP:
Porcine Primary Hepatocytes (PPH) + hNTCP:
Advantages:
Directly isolated from human or animal livers, retaining natural metabolic functions and viral receptor activity.
Ideal for studying HBV replication, drug metabolism, and cccDNA clearance mechanisms.
Limitations:
Short survival time (<14 days), limiting long-term observation of chronic infection.
Complex protocols and high inter-donor variability.
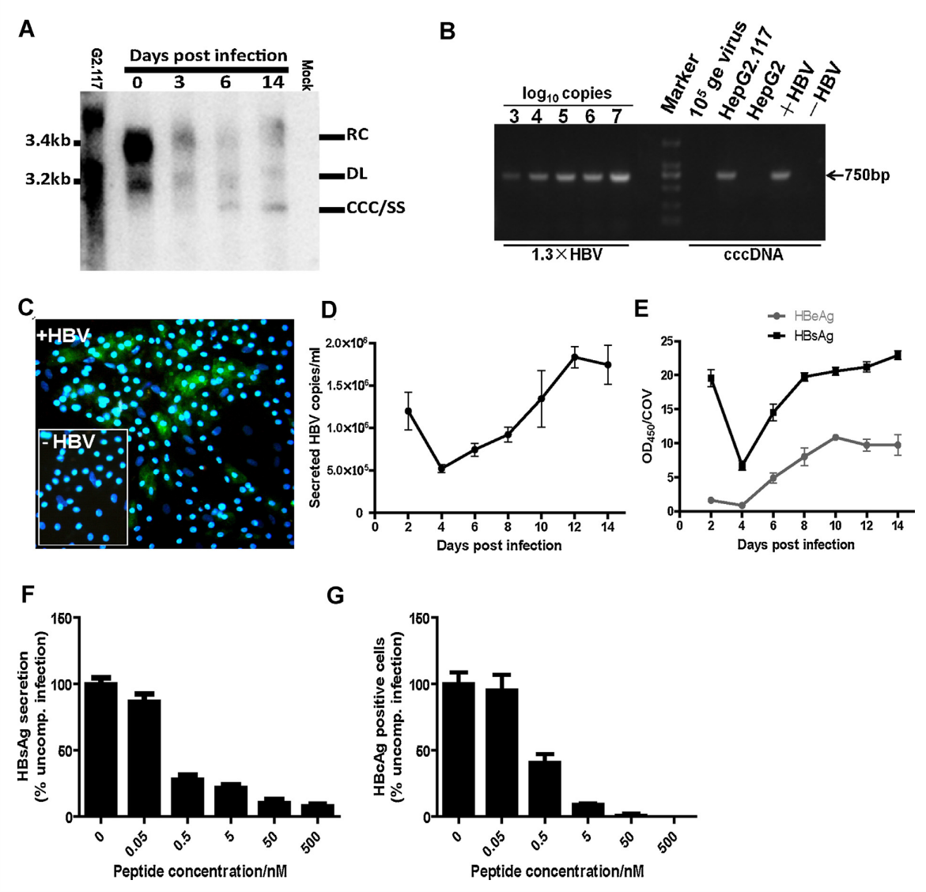
2. Hepatoma Cell Line Models
Common Models:
Key Features:
Advantages:
Limitations:
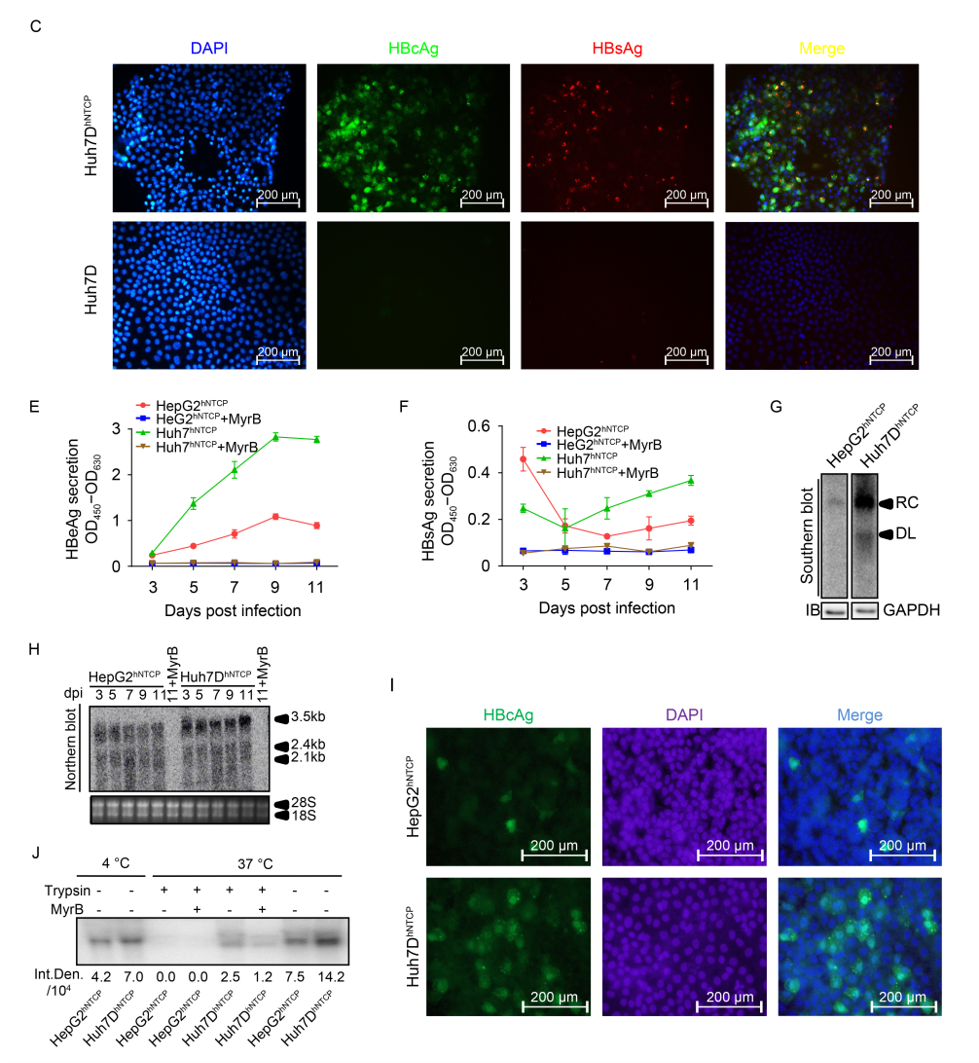
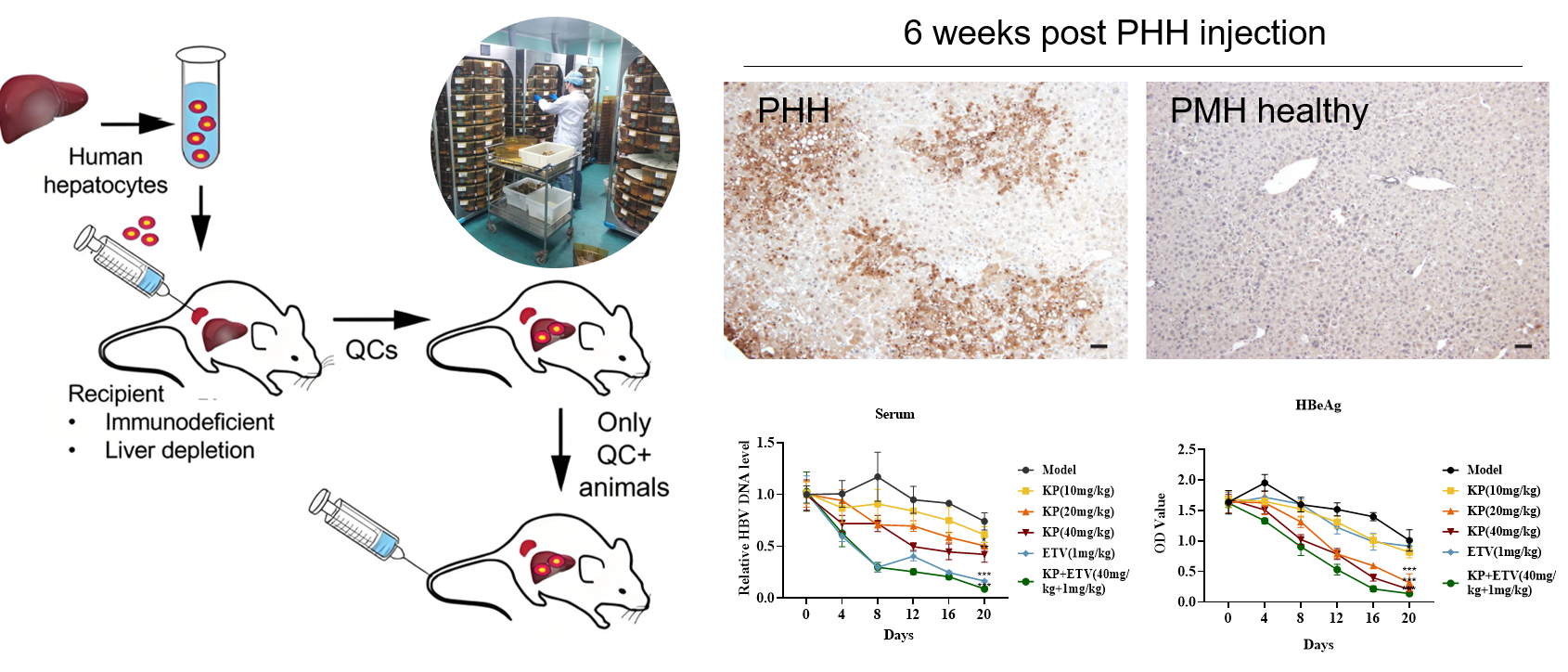
3. Humanized Liver Mouse Models
Humanized liver mouse models are generated by transplanting human primary hepatocytes or liver progenitor cells into genetically modified immunodeficient mice (e.g., FRG, uPA strains), enabling the reconstitution of functional humanized livers. These models support HBV infection, drug metabolism studies, and liver disease mechanism research, and can mimic chronic hepatitis, fibrosis, and other pathological processes. They are widely used for antiviral drug screening and host-virus interaction studies.
Key Features
Advantages:
Recapitulate human liver physiology and support the full HBV infection cycle, including chronic hepatitis, fibrosis, and antiviral therapy evaluation.
Overcome limitations of in vitro models by replicating human liver-specific microenvironments.
Limitations:
High cost and technical complexity in model generation.
Lack of a functional immune system, restricting studies on immune-mediated pathogenesis.
4. 3D Spheroid/mini-liver Infection Model
The 3D spheroid/mini-liver model mimics the liver microenvironment, offering a highly biomimetic platform for studying HBV infection mechanisms and evaluating antiviral drugs. These spheroids are typically constructed by co-culturing primary human hepatocytes (PHH) with Kupffer cells and stellate cells, forming polarized structures and intercellular communication networks.
Key Advantages Over 2D Models
Sustained NTCP expression: Maintains long-term, high-level expression of the HBV receptor NTCP, enabling the complete HBV life cycle (entry, replication, virion secretion).
Stable cccDNA persistence: Supports prolonged stability of HBV covalently closed circular DNA (cccDNA), critical for modeling chronic infection.
Extended culture duration: Viable for up to 2 months (or longer based on experimental conditions), as validated by our team.
Applications
HBV infection dynamics: Study viral entry, replication, and immune evasion strategies.
Antiviral drug screening: Evaluate drug efficacy and resistance in a physiologically relevant system.
Chronic infection modeling: Investigate cccDNA maintenance and therapeutic clearance strategies.
Technical Strengths
Biomimetic complexity: Recapitulates cell-cell interactions, metabolic zonation, and liver-specific functions.
High reproducibility: Standardized protocols ensure consistent spheroid formation and functionality.
Customizable co-culture: Incorporate immune cells or stromal components to study host-pathogen interactions.
Why Choose This Model?
Developed using proprietary 3D culture technology, this platform bridges the gap between simplistic 2D systems and costly animal models, delivering human-relevant data with high translational value
II. Service Credentials, Facilities, and Team
Certified Laboratory Infrastructure
GMP-Compliant BSL-2 Lab: Operates in a Biosafety Level 2 (BSL-2) laboratory registered with the National Health Commission, authorized for handling pathogens including HBV, lentiviruses, and human/animal-derived samples.
Regulatory Compliance: Adheres to strict biosafety protocols and quality management systems.

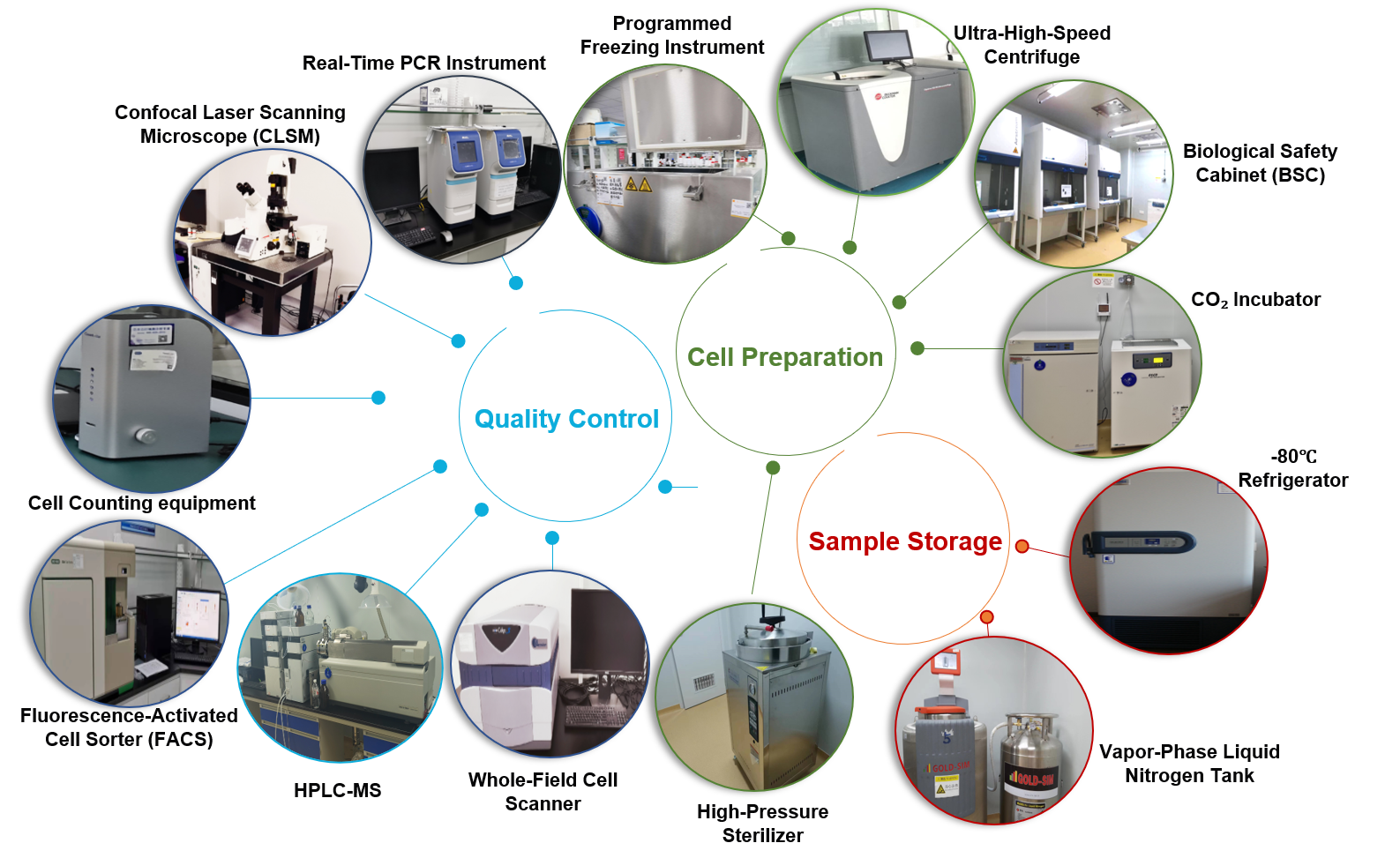
Advanced Equipment and Facilities
Cutting-Edge Instrumentation: Over RMB 20 million+ invested in state-of-the-art equipment, including:
Confocal laser scanning microscope
High-speed fluorescence-activated cell sorting (FACS) systems
Ultracentrifuges
Real-time quantitative PCR systems
Automated cell culture platforms
Dedicated Infrastructure: Climate-controlled workspaces, pathogen containment zones, and advanced imaging suites.
Expert Team
Leadership: Guided by globally recognized scientists, including:
Dr. Ming Zhou – Principal Scientist:
Education: Ph.D. in Virology from the Wuhan Institute of Virology, Chinese Academy of Sciences.
Experience: 14+ years of expertise in HBV pathogenesis and therapeutic development.
Funding: Principal investigator for 7+ national and regional grants, including projects from the National Natural Science Foundation of China, Guangdong Provincial Department of Science and Technology, and Shenzhen Science and Technology Innovation Commission.
Publications: 10+ first/corresponding-author papers in high-impact SCI journals.
Core Competencies
End-to-End Solutions: From model development (e.g., 3D mini-livers, humanized mice) to preclinical data generation.
Translational Expertise: Bridging basic research with clinical applications through physiologically relevant models.
Collaborative Innovation: Partnering with academia and industry to accelerate HBV cure discovery.











