Transport Mode
Liquid Nitrogen Transportation
1、Adsorptive Liquid Nitrogen Transportation ,No free liquid nitrogen ,White vapor emission indicates normal operation.
2、Temperature monitoring devices should closely monitor the temperature inside the tank during transportation. If any abnormalities are detected, you can copy the PDF and Excel data from the temperature recorder. (One end of the temperature monitoring device can be unplugged to reveal a USB connector. Simply insert it into a computer to copy the data. If you encounter any difficulties, you can contact the sales staff and technical support for assistance.)
3、Alloy Combination Lock Designed to ensure no third-party access to the LN₂ tank or cell samples during transportation from dispatch to receipt, thereby safeguarding cell integrity.
4、GPS Tracker Enables real-time tracking of cell transportation routes to prevent loss.
5、Liquid Nitrogen Tank, temperature monitoring device, alloy combination lock, and GPS tracker are returnable components. Please store them properly and avoid damage; failure to comply will result in blacklisting.
Reference Citation
pass over.
Scope of Application
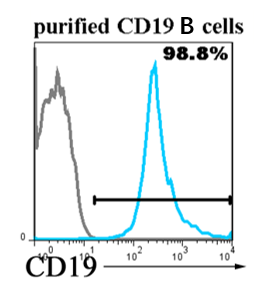
The research applications of human CD19-positive B cells can be summarized as the following core directions:
1. CAR-T Immunotherapy Development
CD19 serves as a critical therapeutic target in B-cell malignancies, including acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL). Through genetic engineering of patient-derived T cells to express CD19-specific chimeric antigen receptors (CAR-T), this approach enables precise elimination of CD19+ tumor cells, establishing itself as a pivotal treatment modality for relapsed/refractory hematological malignancies. To date, multiple CD19-targeted CAR-T therapies (e.g., Yescarta®, Breyanzi®) have received global regulatory approval, with clinical trials demonstrating complete response (CR) rates of 40%-60%.
2. Basic Immunology and Disease Mechanism Research
B cell function analysis: Enrich CD19+ B cells via CD19 magnetic bead sorting technique to study their immunoglobulin secretion (e.g., IgE synthesis), chemotaxis, and signaling pathways (e.g., BCR-dependent Ca²⁺ regulation).
Malignant Transformation Mechanisms: CD19 is continuously expressed from early B cell development to the lymphoma stage. Its complex with CD21 and CD81 is involved in regulating B cell activation and proliferation, serving as a key target for studying the pathogenesis of leukemia and lymphoma.
3. Autoimmune Diseases and Immunoregulation
CD19-targeted therapies (e.g., the monoclonal antibody Uplizna) have been clinically applied in treating autoimmune disorders such as neuromyelitis optica spectrum disorder (NMOSD). Furthermore, investigations into the immunosuppressive functions of CD19+ regulatory B cells (Breg) have unveiled potential therapeutic interventions for systemic lupus erythematosus (SLE), multiple sclerosis (MS), and related pathologies.
4. Drug Delivery System Development
Engineered CD19+ plasma cells can secrete bispecific antibodies (e.g., anti-CD19/CD3) long-term, effectively killing leukemia cells in mouse models. A single infusion maintains drug concentration, significantly outperforming traditional continuous infusion regimens. Such studies provide new insights for the delivery of long-acting biological agents.
5. Diagnosis and Prognostic Evaluation
CD19 expression levels serve as a diagnostic marker for B-cell malignancies (e.g., acute lymphoblastic leukemia [ALL], diffuse large B-cell lymphoma [DLBCL]). Serial monitoring of CD19 positivity enables evaluation of CAR-T therapeutic efficacy and relapse risk. For instance, a pre-treatment CD19 positivity rate of ≥5% is required to meet the eligibility criteria for CAR-T therapy.
Conclusion
The research applications of CD19-positive B cells focus on tumor immunotherapy, disease mechanism analysis, immune regulation, and drug development. Serving as a target or tool cell, they play a central role in both basic research and clinical translation. Future directions include optimizing CAR-T safety (e.g., reducing neurotoxicity), developing dual-target therapies, and expanding indications for autoimmune diseases.
Due to biannual updates of our product instruction manual, the following procedures are for reference only.
The most recent version included with the product shall prevail.
I Reagents and Materials
- Frozen human CD19+ B cells(Cat# LV-Bcell001/002)
- Culture medium(complete RPMI1640.See the table below)
-37℃ Thermostat water bath
- Biosafety cabinet
- Sterile centrifuge tube of 15 ml
- 37 °C/5% CO2 incubator
-Wide-mouth Pasteur pipette and wide-mouth pipette tip
- Pipette
- 75% alcohol
II Complete RPMI1640 Culture Medium
Components | Dosage |
Incomplete RPMI1640 solution | 500ml |
L-Glutamine(L-Glu) | 2mM |
2-Mercaptoethanol(2-ME) | 50μM |
Penicillin | 100μg/ml |
Streptomycin | 100μg/ml |
FCS(Inactivated at 56°C for 30min) | 50ml |
III Resurgence and Plating of Cells
1. Place the complete RPMI1640 medium in a 37 °C thermostat water bath to fully preheated.
2. Quickly transfer the frozen cells from the refrigerated position to a 37 °C thermostat water bath. Immerse them as much water as possible at 37°C and shake horizontally. But make sure the lid of freezing tube remains above the water.
3. Thaw the freezing tube for about 90-120s, until it is just completely dissolved into solution.
4. Sterilize the freezing tube with 75% alcohol and transfer it to a biosafety cabinet.
5. Carefully aspirate the thawed cells and move to the centrifuge tube of 15 ml. Rinse the centrifuge tube and the pipet tip of aspirating cells with medium.
6. Shake the centrifuge tube while adding complete RPMI1640 medium to 12 ml (Note: the first 3 ml should be added dropwise and shaken, and the next 9 ml can be accelerated). Finally, mix upside down and mix well.
7. Centrifuge of 500 × g at 20 °C for 8 min, with ascending to 7 and descending to 4.
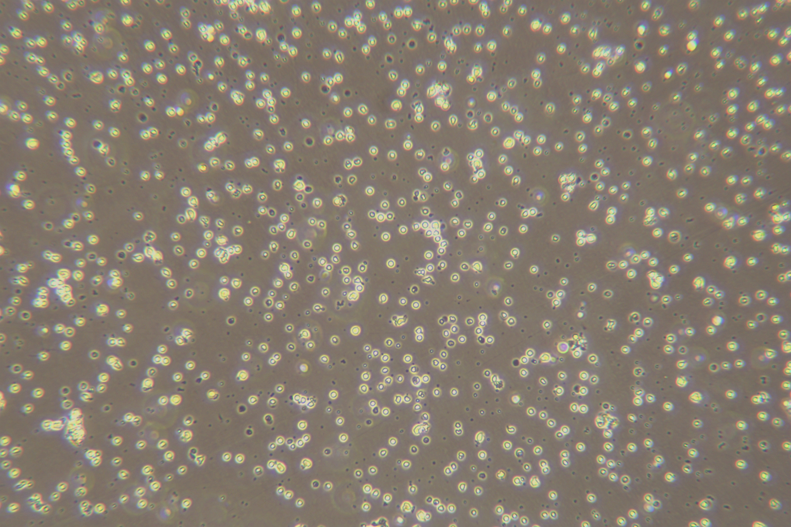
8. Discard the supernatant, add 3 ml of complete RPMI1640 medium to resuspend the cells and count with trypan blue staining.
9. Repeat step 7 again. Resuspend the cells with complete RPMI1640 medium and plate them at the density required by the experiment.
10.Incubate in a 37 °C/5% CO2 incubator.
IV Customer Service
If you find any quality problems with the product, please collect the original data and contact the company's salesmen or technical support at the first time. The company ensure after-sales service. Every laboratory has different conditions, different operating habits, different proficiency, and objective factors in experimental failure. If the operation is not strictly in accordance with the instructions or exceeds the time limit of after-sales, the company does not do after-sales. Please understand and support us.
Validity period and raw data provided:
Resuscitation problems: Within 24 hours of resuscitation, trypan blue staining or PI staining should be provided.
Pollution problems: Within 96 hours of resuscitation, microscope photographs of differences should be provided.
Purity issues: Within one month, immunofluorescence or flow cytometry results should be provided
V Contact Number
Tel:0755-28284050
Technical Support: 19902901483 (Dr. Zhou)