Small Nucleic Acid Drug Efficacy Evaluation
I. Introduction to Small Nucleic Acid Drugs
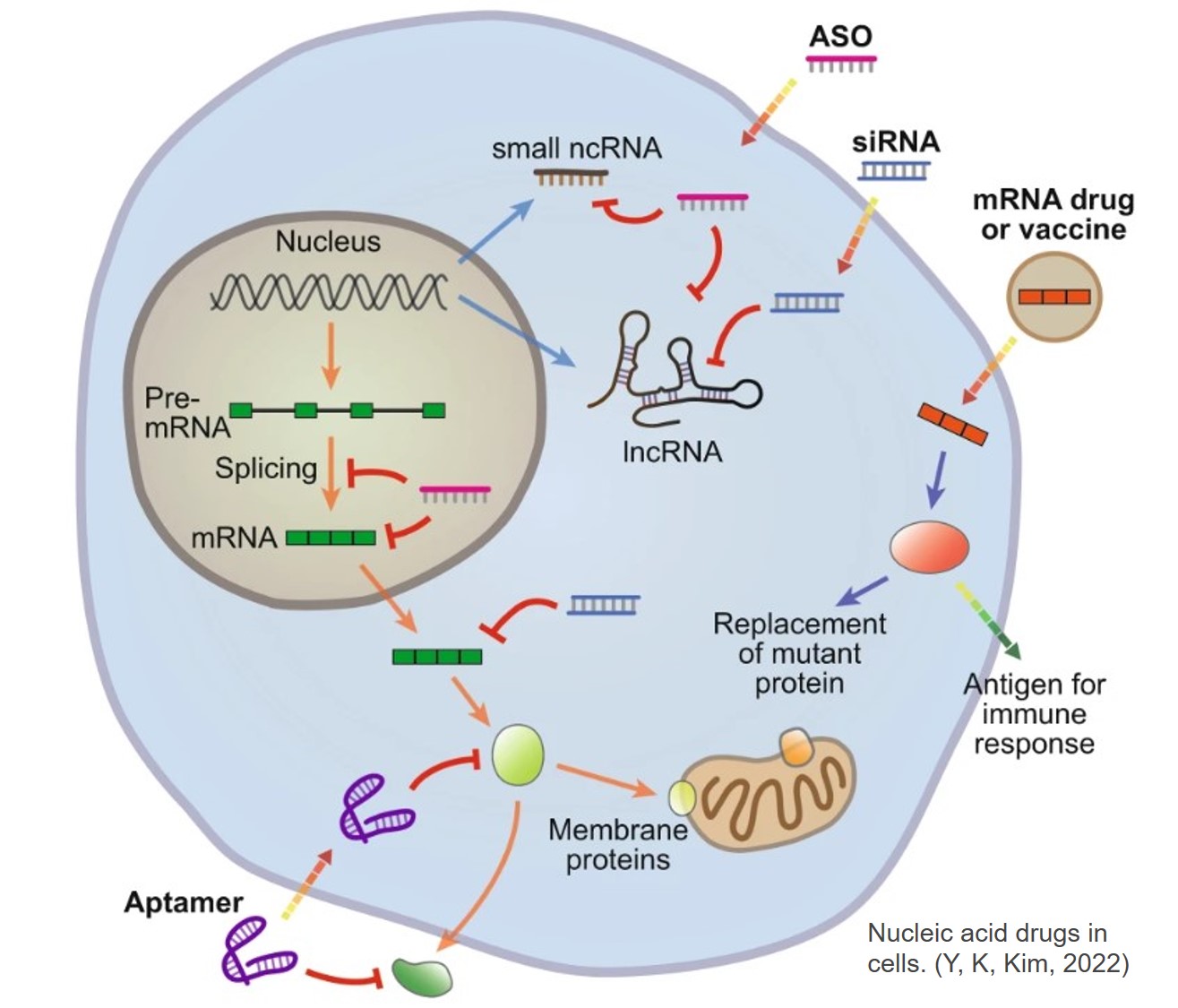
Small nucleic acid drugs are short nucleotide polymers (typically 10–50 nucleotides in length) designed to treat or manage diseases by modulating gene expression. Through base-pair complementarity, these drugs bind to target genes to either silence pathogenic gene expression, correct point mutations, or regulate post-transcriptional processes, thereby achieving therapeutic effects.
Key Types of Small Nucleic Acid Drugs:
Antisense Oligonucleotides (ASO)
Small Interfering RNA (siRNA)
MicroRNA (miRNA) Mimics/Inhibitors
Aptamers
Unique Advantages:
Broad Target Potential: Virtually any gene can be targeted with a synthetically designed nucleic acid sequence, overcoming limitations of traditional small molecules and antibody-based therapies.
High Specificity: Precise targeting minimizes off-effects.
Durable Efficacy: Long-lasting gene silencing reduces dosing frequency.
Low Toxicity & Resistance Risk: Reduced likelihood of adverse reactions or drug resistance.
Rapid Development: Streamlined design, synthesis, and scalable production.
High Clinical Success Rate: Strong translational foundation with applications in rare diseases, chronic disorders, and oncology.
Clinical Breakthroughs:
Inclisiran: The first siRNA drug targeting LDL-C levels, approved by the EU (Dec 2020) and FDA (Dec 2021) for treating hypercholesterolemia and mixed dyslipidemia.
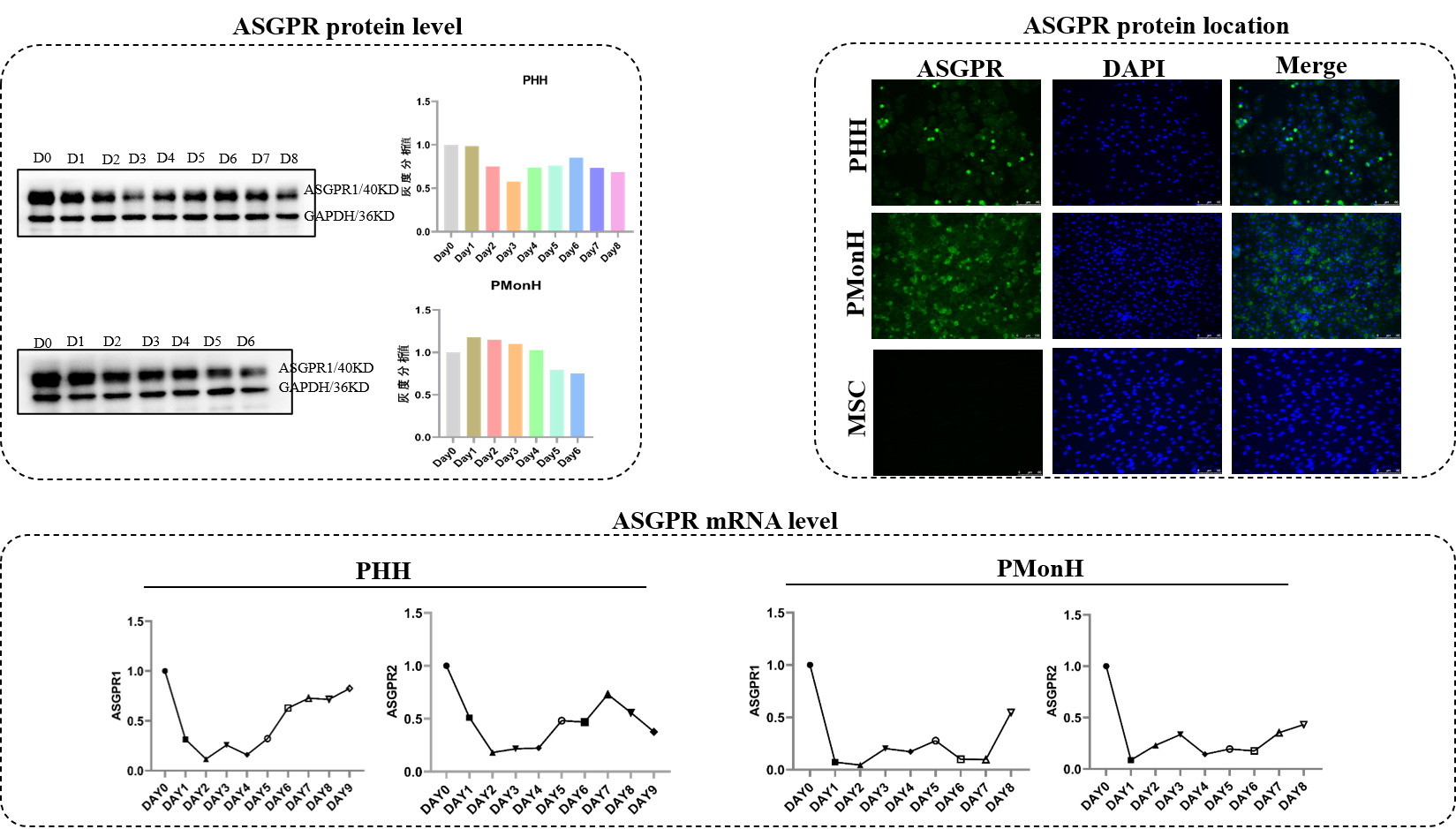
Liver-Targeted Delivery: Emerging focus on ASGPR-GalNAc conjugate systems to enhance hepatic delivery efficiency, driving innovation in liver disease therapeutics.
II. Model Overview
1. 2D Primary Hepatocyte Model
Primary hepatocyte culture is regarded as the "gold standard" for in vitro liver models, widely used for drug and toxicology studies. In this system, primary hepatocytes are seeded on a solid-phase extracellular matrix (e.g., collagen) for monolayer culture, preserving most liver-specific functions.
Advantages:
Physiological Relevance:
Enzymes and cofactors in primary hepatocytes exist at natural physiological concentrations, enabling drug metabolism and toxicity studies under near-native conditions.
Maintains intact cellular morphology and metabolic activity, accurately reflecting uptake, transport, and metabolism of small nucleic acid drugs.
Species Diversity:
Human, monkey, dog, pig, rabbit, rat, mouse, cow, sheep, donkey, chicken, duck, goose, and fish.
Liwo Bio offers 14 species-specific 2D primary hepatocyte models, covering:
Applications:
Drug Metabolism Studies: Evaluate hepatic clearance, metabolite profiling, and enzyme induction/inhibition.
Toxicity Screening: Predict hepatotoxicity and off-target effects in a human-relevant system.
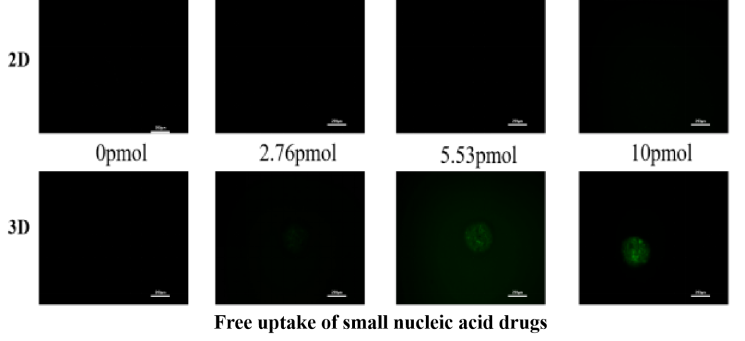
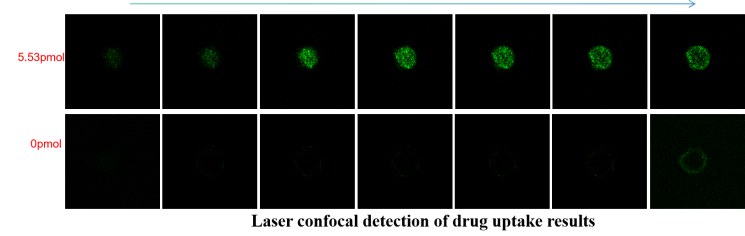
Nucleic Acid Drug Development: Assess liver-targeted delivery efficiency and therapeutic activity.
2. 2D Liver Non-Parenchymal Cell Models
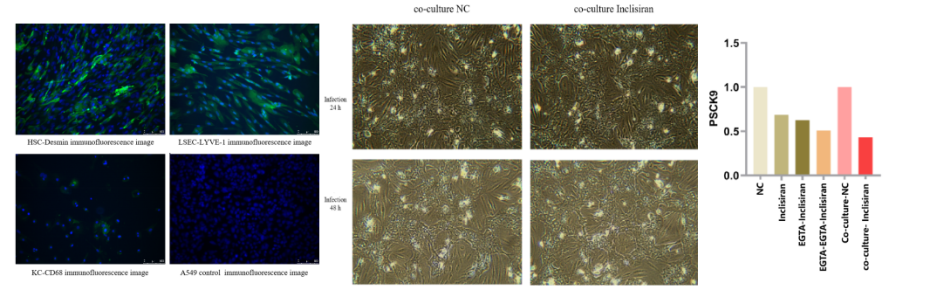
Liver non-parenchymal cells (NPCs) are non-hepatocyte cell types that play structural, immune, and regulatory roles in liver physiology. These cells support hepatocyte function and participate in disease pathogenesis. Key subtypes include:
A. Liver Sinusoidal Endothelial Cells (LSECs)
Structural Role:
Form a fenestrated endothelial barrier lining liver sinusoids, facilitating nutrient exchange between blood and hepatocytes.
Promote hepatocyte access to blood-borne nutrients and metabolites.
Functional Significance:
B. Kupffer Cells (KCs)
Role in Innate Immunity:
Liver-resident macrophages with high motility, critical for host defense and immune tolerance.
Engulf pathogens, cellular debris, and toxins (e.g., bacterial endotoxins).
Disease Relevance:
Alcohol-induced liver disease (ALD)
Non-alcoholic steatohepatitis (NASH)
Drug-induced liver injury (DILI)
Produce pro-inflammatory cytokines (e.g., TNF-α, IL-6) that mediate hepatoprotection or exacerbate injury in conditions like:
Drive fibrogenesis by activating HSCs in chronic liver injury.
C. Hepatic Stellate Cells (HSCs)
Applications in Drug Safety Assessment
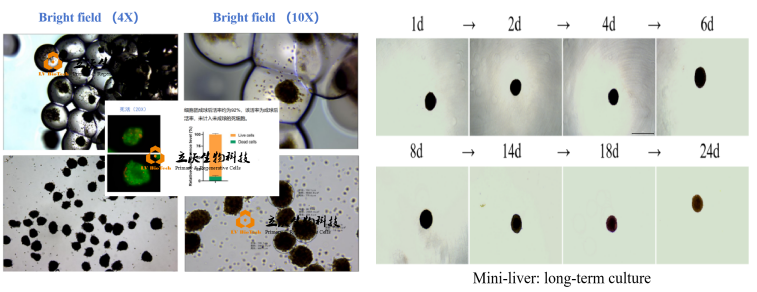
3. 3D Spheroids (Mini-liver)
The 3D spheroid/mini-liver model is generated by co-culturing primary hepatocytes and liver non-parenchymal cells (NPCs) in precise ratios within ultra-low attachment plates. Through self-assembly, these cells form spheroids within 24 hours, enabling long-term culture (weeks to months) while maintaining high functionality.
Key Advantages
Technical Highlights
Rapid Self-Assembly: Proprietary ultra-low adhesion technology ensures spheroid formation within 24 hours.
Sustained Functionality: Optimized culture media maintain hepatocyte polarity and metabolic activity for ≥2 months.
Translational Precision: Bridges gaps between 2D models and in vivo systems for human-relevant data generation.
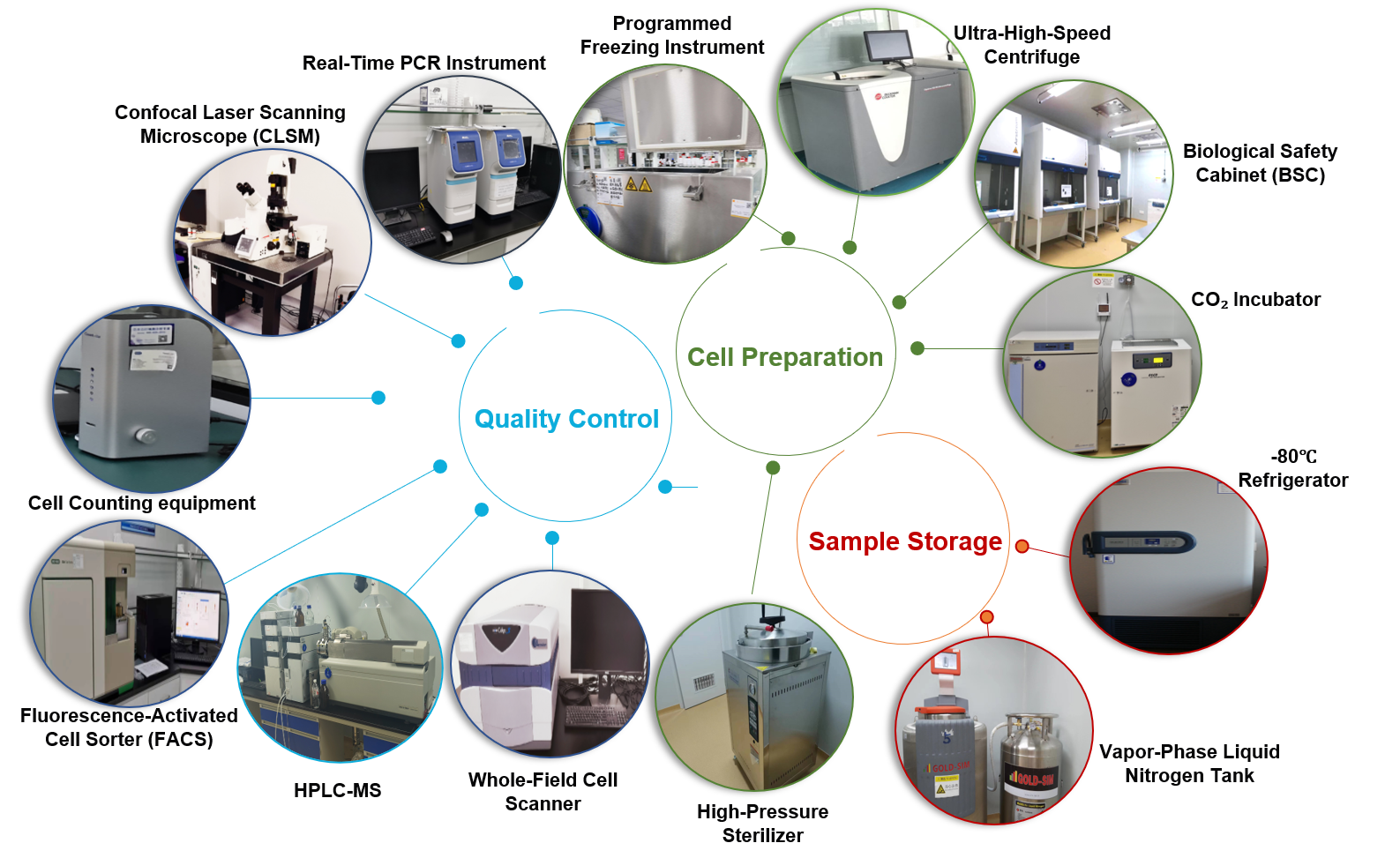
III. Service Credentials, Facilities, and Team
Certified Laboratory Infrastructure
GMP-Compliant BSL-2 Lab: Operates in a Biosafety Level 2 (BSL-2) facility registered with the National Health Commission, authorized for handling pathogens including HBV, lentiviruses, and human/animal-derived samples.
Regulatory Compliance: Adheres to stringent biosafety protocols and quality management systems.

State-of-the-Art Equipment
RMB 20+ Million Investment in advanced instrumentation:
Confocal laser scanning microscope
Fluorescence-activated cell sorting (FACS) systems
Ultracentrifuges
Real-time quantitative PCR (qPCR) systems
High-throughput screening platforms
Dedicated Workspaces: Climate-controlled labs, pathogen containment zones, and automated cell culture suites.
Elite Scientific Team
Core Strengths
Integrated Solutions: From preclinical model development (e.g., 3D spheroids, humanized mice) to mechanistic drug evaluation.
Translational Excellence: Combining cutting-edge models with clinical insights to accelerate therapeutic discovery.
Collaborative Innovation: Partnering with global academia and industry to tackle unmet needs in liver disease research.
IV. Service Case Studies
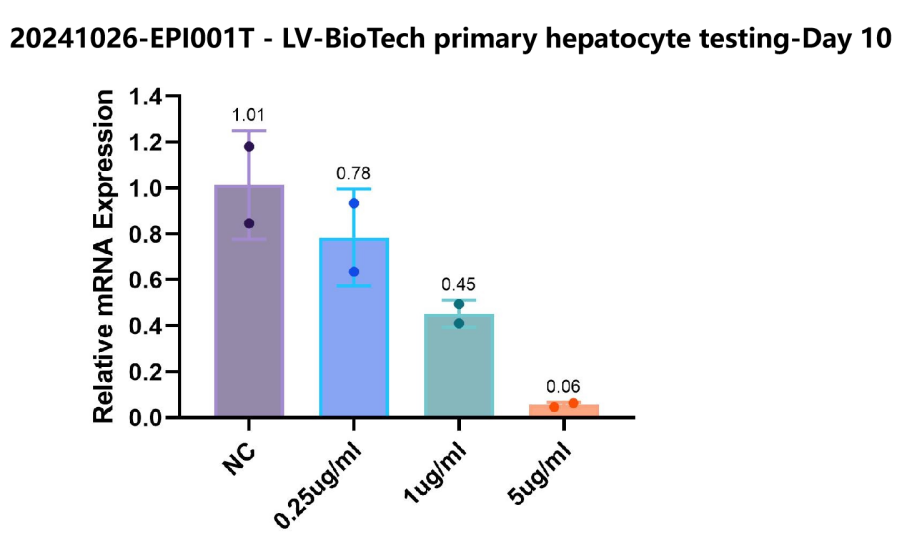
Case Study 1: Inclisiran’s Impact on PCSK9 Expression
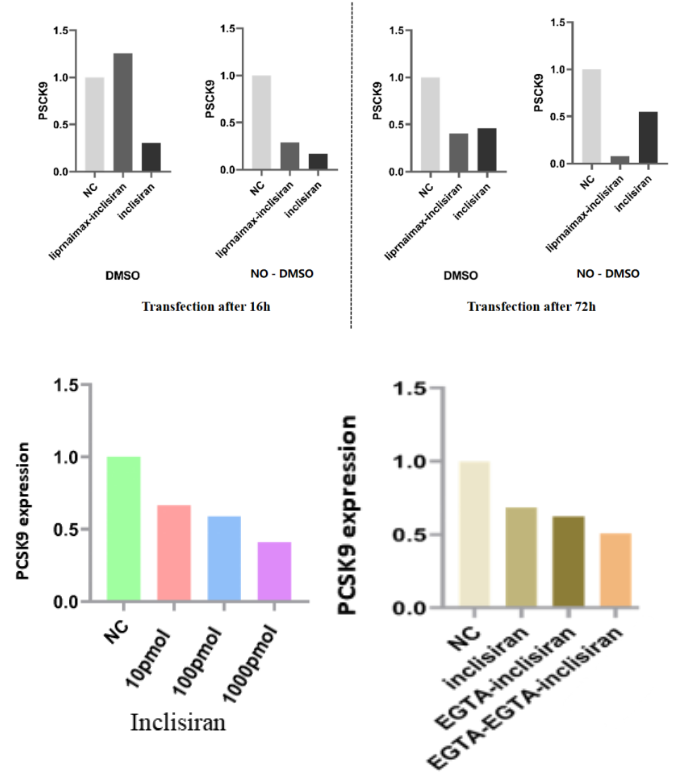
Case Study 2: Knockdown Efficiency of a Positive Control Drug on [Target Gene X]
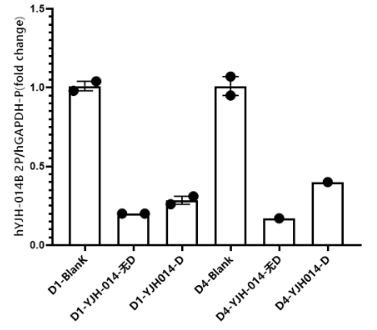
Case Study 3: Evaluation of a Novel Nucleic Acid Drug on [Target Gene Y]